It is not a secret that information technologies (IT) permeate virtually every kind of human activity. The domain of drug research and production is not an exception. Generally speaking, IT can revolutionize data collection, storage, and processing in pharma. Only cutting-edge IT can drive disruptive innovation in pharma, and companies that preferred to stick to traditional workflows and practices have already started to feel the difference.
Types of innovation in the pharmaceutical industry
Our article will mostly focus on digital innovation in pharma, although there are many possible meanings that innovation could have within the given context.
For instance, concerning the development of medicinal drugs in academic or corporate environments, there are two types of innovation: radical and gradual.
The radical type is remarkable for inventing the kind of drugs that represent a whole new therapeutic class in the best-case scenarios. Radical innovation is attractive because it guarantees no immediate competition in the foreseeable future, but it is also quite expensive and time-consuming.
The gradual type, on the other hand, entails modifying an existing drug (including the ones obtained through radical innovation) and improving its quality (safety, effect duration, effectiveness, and such). Gradual innovation is a safer bet compared to the radical one.
Both types have their own distinct marketing strategies, and the latter, in turn, are subject to innovation as well.
So, to reiterate, we will leave the aspects that do not relate to information technology aside for now.
Top digital innovation trends in pharmaceutics
As a side note, the difference between pharmaceutics and pharmacology is that the former deals with research, production, and distribution of drugs while the latter studies the drugs’ impact on the body. That settled, let’s move on.
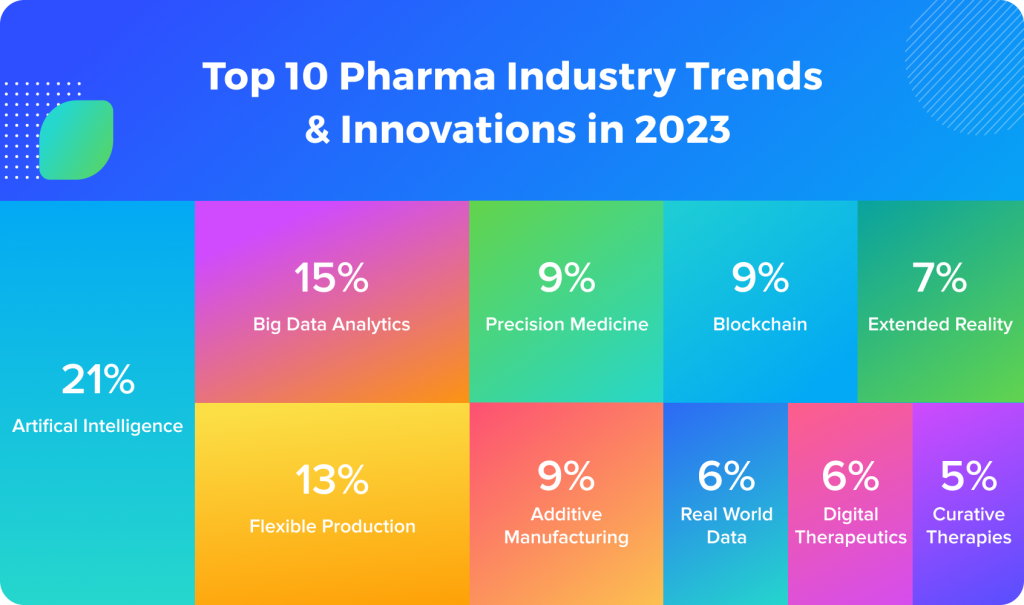
Source: StartUs Insights.
Artificial Intelligence (AI) and Machine Learning (ML)
In a nutshell, AI applications are designed to analyze large data sets and come up with practical insights that would take hours or even years of human labor. AI usually uses the known connections between raw data and practical knowledge as its learning data set.
In the pharmaceutical industry, AI might be involved on the stage of research and development (R&D) to outline potential candidates for a radically new drug from a huge set of possible variants, basing on its desired pharmacological properties, or suggest improvements for an existing one.
AI is also known to be highly efficient in diagnosing illnesses and suggesting treatments. Given a large enough database of patients, pharma companies may fine-tune their targeted ads and come up with best possible personal therapy suggestions with the power of AI.
The abovementioned capabilities of AI to both identify a disease and suggest a drug treatment that may not even exist yet imply its usefulness in rare disease treatment. Considering that the drugs that could treat rare diseases see a relatively low demand, their development is too often not well-justified financially.
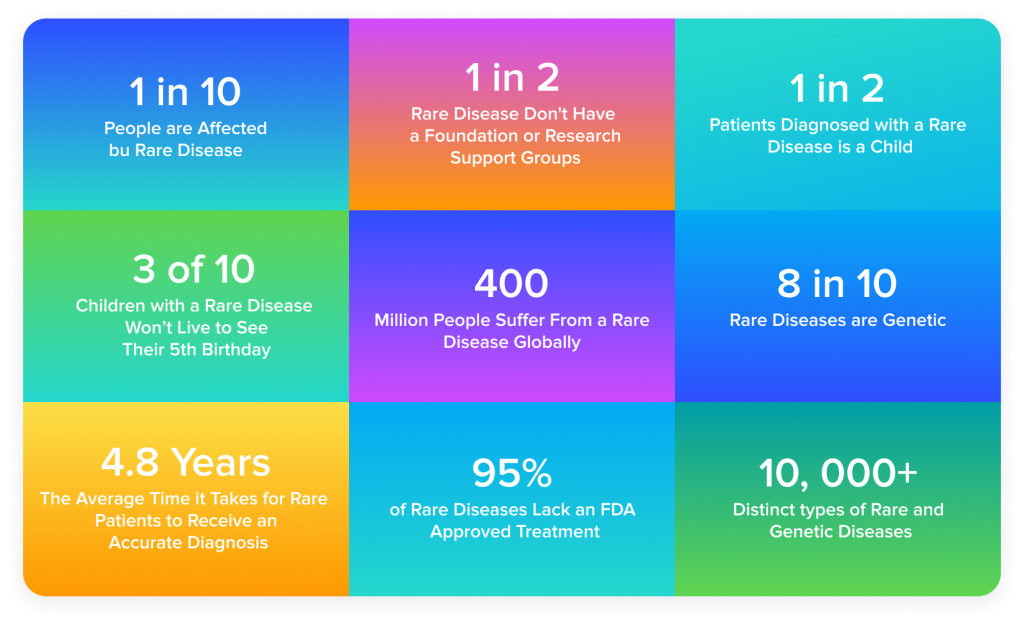
Source: GlobalGenes.
Not only can AI predict individual predisposition to certain diseases based on a patient profile, but also collect and analyze data in real-time though wearable devices. Should an owner of such a device give consent to track their body’s biometric data, it can warn them of an increased disease risk long before its symptoms start to emerge.
It can predict epidemic outbreaks as soon as it detects enough leading indicators from available sources, too. When an AI system issues an outbreak warning, pharmaceutical companies can direct the effort into preventing it and mitigating its possible consequences.
Some of the more trivial and widespread AI use cases include image and video analysis, speech recognition, text-to-speech, intelligent chatbots, personalized recommendations, target audience segmentation, and others.
By the way, eWizard team is working to integrate Amazon Web Services (AWS) AI/ML tools so that some of these functions are available out-of-the-box.
Big Data and Internet-of-Things (IoT)
Big Data and Artificial Intelligence technologies go hand in hand, but the former does not always imply the use of the latter.
Big Data is focused on handling and processing large amounts of data while AI is focused on using that data to build intelligent systems that can automate tasks, predict outcomes, or provide insights.
AI does rely on large amounts of data to learn and make predictions, but not all Big Data projects involve AI. In some cases, Big Data may be used for simple data analytics or reporting purposes without involving state-of-the-art AI systems.
The question though, how and why would pharmaceutical organizations collect, store, and process unimaginable loads of data? Any purpose we mentioned before may apply: identification and segmentation of potential or existing target audiences, prediction and prevention of personal diseases and outbreaks, feedback collection and experience personalization, to name a few. As for the data collection means, the IoT technologies come into play: certain IoT devices can collect and send biometric data. For instance, automatic acute stroke symptom detection system is backed by these technologies.
Virtual Reality and Augmented Reality (VR/AR) use cases in pharma innovation
The technologies related to mixed reality, virtual reality, and augmented reality are sometimes generally referred to as Extended Reality (XR).
Augmented Reality headgear is semi-transparent, allowing its user to see both the real environment and the VR overlay depending on the context, whereas Virtual Reality headgear completely isolates the user from the environment.
XR technologies deal primarily with visual representations of certain data. They are increasingly more popular in education, and the pharmaceutical industry could widely implement them as well. For instance, Nanome revolutionize drug design by visualizing complex inorganic molecules and proteins and enabling a chemist to work with their models in real time, also providing the tools to measure their properties.
XR also greatly increase the efficiency of learning, training, and collaboration. Solo professionals may improve their productivity, safety, and the error rate with XR work-related tips.
Blockchain
During the last decade, blockchain was mostly popularized by cryptocurrencies, so there might be a misconception that it lacks other applications than FinTech. Truth is, blockchain is a technology that ensures safe decentralized storage of all kinds of data, making the data incredibly resistant (practically immune) to alteration.
Accompanied with advanced encryption technologies, blockchain also ensures private data exchange, so that the identities of involved parties remain protected. Considering that both patient data, research data, and financial information all classify as highly sensitive information, no wonder that the pharmaceutical industry at large is getting interested in blockchain.
Another feature that can be built on top of blockchain is smart contracts. What it does is basically track the contract execution conditions so that it does not have to be signed manually when the conditions are met. Smart contracts deal with real-world data that must first be gathered and written to the chain.
Supply chain tracking is one of the most important use cases of blockchain and smart contracts in pharmaceutical innovation. It enables ingredient tracking from suppliers to drug manufacturers, to distributors, all the way to the consumers. It is crucial for anti-money laundering / know-your-customer (AML/KYC) practices and literally saves lives by keeping counterfeit drugs away from the supply chain. Pharmatrace is a good example of blockchain application in pharma.
Pharmaceutical industry innovation in production, dosage, and packaging
IT is usually at the core of production innovation in the niche in question, but the connection between pharma and IT is different in this case. Pharmaceutical innovations that deal with manufacturing, dosage, and packaging rely on advanced machinery that, in turn, utilizes the software that corresponds to its application.
Production innovation in pharmaceutics
The industry is extremely reliant on fast upscaling and downscaling, and, for that matter, production methods are a sensitive issue. On top of that, mass production excludes the possibility to produce personalized medications.
Precision medicine is a healthcare approach that recognizes and emphasizes the uniqueness of each patient’s biological makeup, environmental influences, and lifestyle factors. The goal of precision medicine is to develop targeted treatment plans that take into account an individual’s specific medical condition, genetic traits, and personal health history, rather than adopting a one-size-fits-all approach.
Advanced manufacturing methods, such as additive manufacturing or 3D printing, have made it possible to create precision pills with delayed or suspended action mechanism, polypills (multidrug combination pills), and drug delivery systems adjusted to individual patients’ needs. It is exactly what FabRx does.
Single-use bioreactors are particularly useful in biopharma: they provide perfect control over production volume, allow regulation of the batch size, and remove the necessity for repetitive cleaning and disinfection.
AI applications may be involved at any stage of production line design or optimization, effectively saving costs, production times, and materials.
Packaging innovation of pharmaceutical products
The motivation behind pharma packaging innovation is straightforward: cheaper, faster, and more sustainable packaging production. From the standpoint of consumers’ needs and expectations, a package should be safe and convenient, enable self-administering and fine dosage regulation, also being easy to dispose of, recycle, or degrade.
When it comes to cost-efficiency, 3D-printing technologies are worth mentioning again: they simplify production of custom packing designs in low volumes at low price, which is great news for pharmaceutical startups.
The use of sensors opens up possibilities to design smart packaging that tracks storage conditions of a given drug, its expiry date, and other relevant data. It increases patient safety by preventing the use of expired, improperly stored, or counterfeit drugs.
Another pharma tech innovation direction is sustainable packaging design. Pollution is one of the major concerns of ethical businesses, so it goes without saying that zero-waste production is the goal of any such organization. Recyclable and biodegradable packs represent the steps in eco-friendly development.
There are also initiatives for developing child-resistant packaging so that certain drugs could not be accidentally applied or ingested by children.
Marketing innovation in the pharmaceutical industry
A respectful attitude to clients lies at the core of any successful marketing strategy, particularly if a given company aims to build long-term relations and trust with them. At the stage acquisition, that entails studying the target audience’s needs and active requests to deliver a corresponding product or service. At the stage or retention, that entails distributing personalized targeted ads that they are likely to be genuinely interested in.
Viseven eWizard team proudly admits that their product is a part of pharmaceutical industry innovation in the domain of marketing: eWizard automates or simplifies all kinds of tasks related to promo content creation, approval, and distribution, in a highly technological manner.
eWizard features a ton of integrations with third-party service providers so that it can serve as headquarters of all content operations.
The recent advancements that eWizard helps pharmaceutical companies to implement include content modularity, omnichannel marketing, and content auto-tagging, which, taken together, saves tons of time for sales and marketing teams.
Issues with innovation in the pharmaceutical industry
One of the most pressing issues is the high cost of drug development, which can deter companies from investing in risky research and development projects. Another is the lengthy and complex regulatory approval process, which can delay the launch of new drugs and result in significant expenditures.
At times, digital innovation is not even on the list of a company’s immediate priorities. Only being pressed by challenges like COVID-19 or fierce competition, such a conservative company embraces change and eventually becomes an advocate of innovation. Besides, the industry is heavily regulated and reliant on legacy systems and processes, so innovation is generally slower here than in most other domains.
It is never too early and never too late to innovate. Adopting the best industry practices, implementing cutting-edge technologies, and using advanced equipment is always beneficial for all sizes of pharma companies in the long run.
