It is time that we notice a pink suede elephant standing in the middle of the room. Content development in pharma is super-challenging, whether you are new to the field or an experienced marketer. Numerous digital platforms, data siloes, regulations, and time-consuming MLR reviews make it a serious and unique challenge compared to other industries.
You need a clear understanding of the process and practical strategies to ensure your content moves forward and complements the goals of the business. In this blog post, the eWizard team will guide you through each phase of the content development process to help you produce engaging content on time and step up your content strategy.
What is Content Development?
Content development is planning, creating, and distributing content across markets and channels to attain content marketing goals. With a systematic approach, teams are likelier to stay on track with their objectives and deliver high-converting content that meets the audience’s real-time needs.
Content development in pharma can be divided into seven stages: crafting a data-driven content strategy, creating a central repository, producing content, undergoing MLR, localization, tracking performance, and refining campaigns.
Efficient Content Development Process in 7 Steps
Let’s discuss these phases of the content development process in more detail.
Crafting a data-driven content strategy
If the goal of your content development strategy is omnichannel engagement, it is crucial to align your content plan with your channels and customer touchpoints. Start by asking yourself simple questions like:
- Who is my target audience?
- Can I repurpose any existing content?
- Is my data siloed?
- How can I connect my digital and physical channels to create a seamless customer experience?
Without a data-driven content development plan, your teams will likely feel disoriented like a deer caught in headlights. So, you need to understand what data is available to you and how you can leverage it.
Then, think about how you will distribute this data and to whom. The answers to these questions will allow you to identify stakeholders, deliverables, and key content development milestones.
Creating a central repository
A content repository or Digital Asset Management (DAM) system stores the massive amounts of data pharma companies produce daily. By centralizing this data, it breaks down silos that often limit collaboration between global, regional, and local teams.
It also enables pharmaceutical companies to reuse and repurpose their assets across different markets and channels. And with that comes greater scalability and efficiency in their marketing campaigns.
Yet, with all this data in one place, a key challenge arises: How can a company quickly find the right asset in an ocean of information? Centralized content tagging lets users easily find and select the assets or modules they need when they open the DAM system.
It is more like walking into a supermarket. You never feel lost despite its size because the overhead signs guide you exactly where you must go.
In our experience, many pharmaceutical companies shy away from adopting central repositories because manual tagging is labor-intensive. We recommend leveraging artificial intelligence (AI) to automate this process. For example, our content experience platform, eWizard, enables auto-tagging, which is ten times more cost-effective and 100 times faster than manual efforts in terms of both accuracy and performance.
Producing pharma content
Life sciences brands must produce enough content to meet growing customer demands. The modular content approach is one of the most effective ways to scale the content development process without sacrificing quality.
Modular content development involves breaking assets into meaningful chunks or modules that can be reused and repurposed. This means a) there is no need to go through MLR approval each time, and b) you can track the performance of individual modules and reuse high-performing ones in future campaigns.
There are two main ways to implement modular content. The first involves breaking down existing content to find key themes, elements, or structures. Intros, calls to action, disclaimers, or images can be transformed into modules and templates for future reuse.
The second method focuses on setting up the content development process with modularity in mind from the get-go. This requires teams to adopt a new mindset and skill set. Marketers need to think in terms of separate, meaningful content chunks and write in a modular way. It is important to maintain consistent grammar and style, avoid dressing up your vocabulary, and remove unnecessary details from images (like text or labels).
Undergoing MLR approval
MLR approval is often seen as the biggest bottleneck in pharma marketing. And we will not argue with this. The review process by MLR teams can take up to two months, and if they spot any errors, you are back where you started. That is not ideal because every delay means missed opportunities.
At this point, the best way to avoid revisions and content delays is to ensure your marketing material is error-free. AI-powered eWizard can help by spotting issues with context, grammar, references, or image suitability. Moreover, the tool suggests ways to fix them and shows the likelihood of content approval. Users gain confidence and peace of mind when submitting their content for approval.
Large pharmaceutical companies face an even more complex process. They must secure MLR approval at both global and local levels while also managing version control. eWizard lets them easily track approval statuses.
This is how it works: red means the content has not been approved at either level, yellow shows global approval, and green means it is fully approved. If any changes to the document are made, all statuses are reset.
Localizing content
Modern AI tools can automate most of the translation process. If you are concerned that a model does not understand your industry-specific or company-specific terms, the good news is it learns and improves over time. The more content you provide, the smarter and more accurate it becomes, speeding up the content development process.
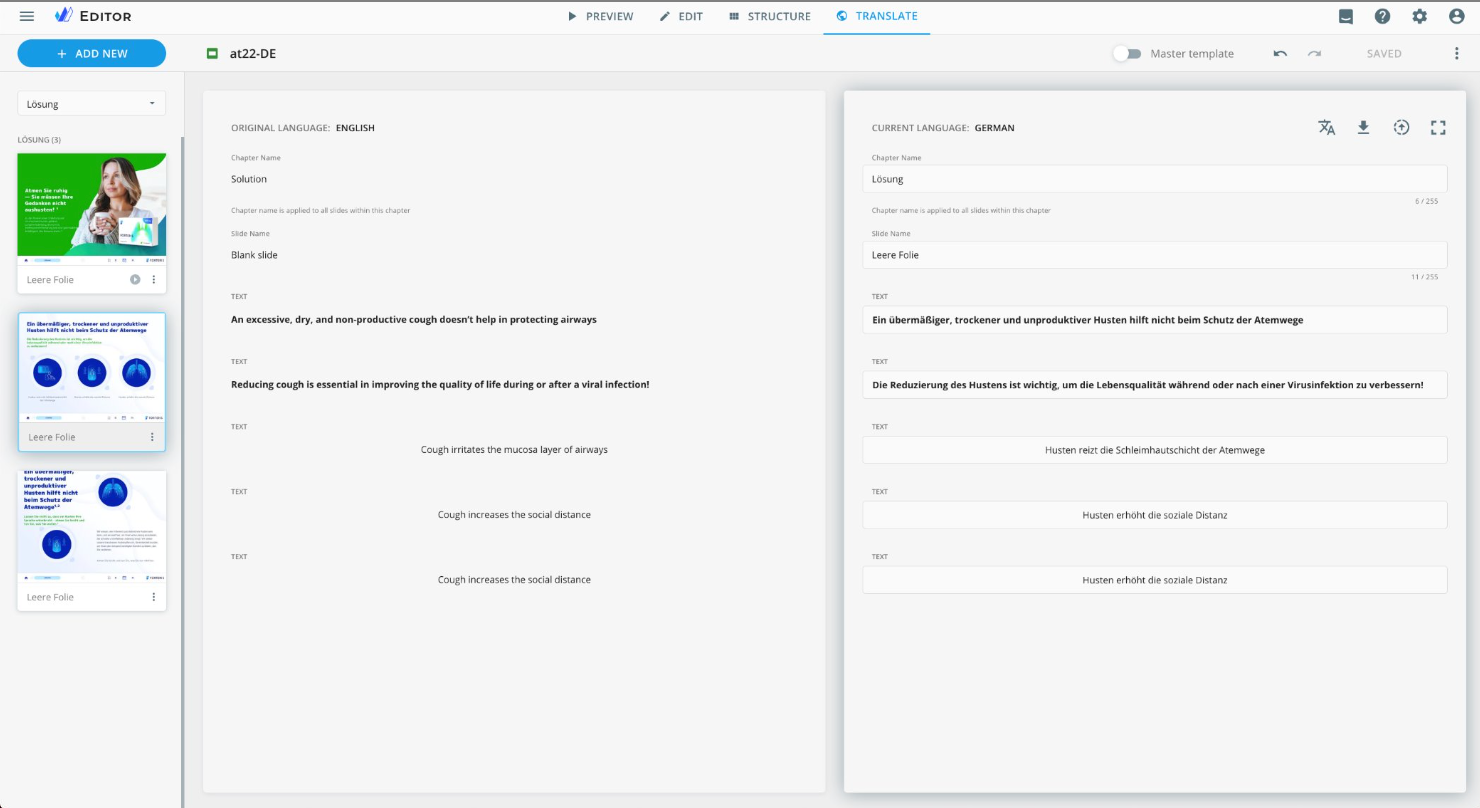
eWizard auto-translation feature
When it comes to localizing individual modules, there is some bad news. Our data shows that local markets prefer adapting the entire content rather than separate modules. As a result, less than 2% of local content is reused.
We have disrupted the traditional modular content approach to boost reuse rates at the local level. eWizard’s new process allows local teams to continue localizing the full content as they are used to. If global content contains modules, they can add the correct metadata and watch the tool localize them automatically in mere seconds.
Deploying content across channels and measuring impact
Data should drive not just the content development process, but content deployment as well. Understanding when customers are most receptive and which channels they prefer is becoming increasingly important.
Tools like Einstein Send Time Optimization help pharma companies identify the optimal time to send messages to each individual HCP. They collect engagement data from HCPs and send users a push notification when the timing is right and they are most receptive to incoming emails.
Once your content is live, tracking its performance helps determine whether you are meeting your goals. With our centralized tagging solution, we can monitor not only the performance of the entire content but also individual sections and modules.
This precise data analysis helps identify which combinations of marketing materials yield the best results and drive greater engagement. The goal is to build a library of high-performing modules that enhance personalization at a scale. That way, marketers can further improve the content development process by reusing the most impactful modules and then sending the messages to the right people at the right time.
Optimizing content
It is crucial to see content development as a flexible, ongoing process rather than something set in stone. You can use analytics insights to find opportunities for improvement or to create even more engaging content marketing campaigns in the future.
Data visualizations are incredibly useful here, helping you make sense of large amounts of raw information. Once you have analyzed which modules or assets are performing best, you can mix, match, or reuse them across different platforms or markets to boost engagement.
Are There Any Common Mistakes?
Content development in pharma is a long journey with plenty of potential pitfalls. It is best to be aware of them well in advance. Here is the list of common content marketing mistakes to avoid:
Creating campaigns from the top down
People are not afraid of change itself. They are worried about not knowing where they fit in afterward, and the changes in the current content development process can cause some unwarranted anxiety among content marketers. When changes are communicated in a top-down manner, that fear can skyrocket.
This often leads to a ‘not-invented-here mindset’ where employees push back on new roles and see their leader as the only decision-maker. The fundamental mistake is introducing a content development strategy and only looping in teams at the implementation stage. People are much more open to new approaches when they are involved in co-creation from the start.
An easier way to manage change is by using digital collaboration tools. eWizard Planner, for example, helps pharma teams collaborate, plan budgets, and track implementation in real time. When everyone has a chance to helm the ship and suggest ideas, teams no longer feel like order-takers – they feel heard and appreciated.
Making all content modular
Many companies mistakenly believe that once they have gone modular, every piece of legacy content needs to be modularized as a part of the new content development efforts. When they start seeing the initial benefits of modular content, it is natural to want to get more out of it. While that is understandable, it can create unnecessary disruption in the existing content development process.
Modules without clear use cases can clutter your DAM system, making navigation hard. As teams will struggle to find the modules, the overall reuse rate will drop significantly.
When developing a content marketing strategy, teams must align on what is worth modularizing. Tracking content performance helps pinpoint which pieces should be broken down further. Regular audits can also help get rid of modules that are no longer performing well.
Disregarding a design element
Many content marketing teams overlook the design aspect when creating modules. And we get why. Since modules are often considered design-less units, marketers may wonder why they should bother with design.
However, in practice, modules do not stay ‘bare’ once they are in channels. Ignoring design leads to inconsistent user experiences for customers and low engagement for brands.
Yet, when marketers consider design, they often must memorize all the modules to pair them with the right blocks. This can be especially tough for new hires or in large global companies with a vast number of modules in storage.
eWizard users, however, do not have this issue. They can focus on creative and engaging content creation without much housekeeping involved. Our universal block layout makes it easy – you must choose the modules you need, and the system automatically suggests the perfect block. It is that simple.
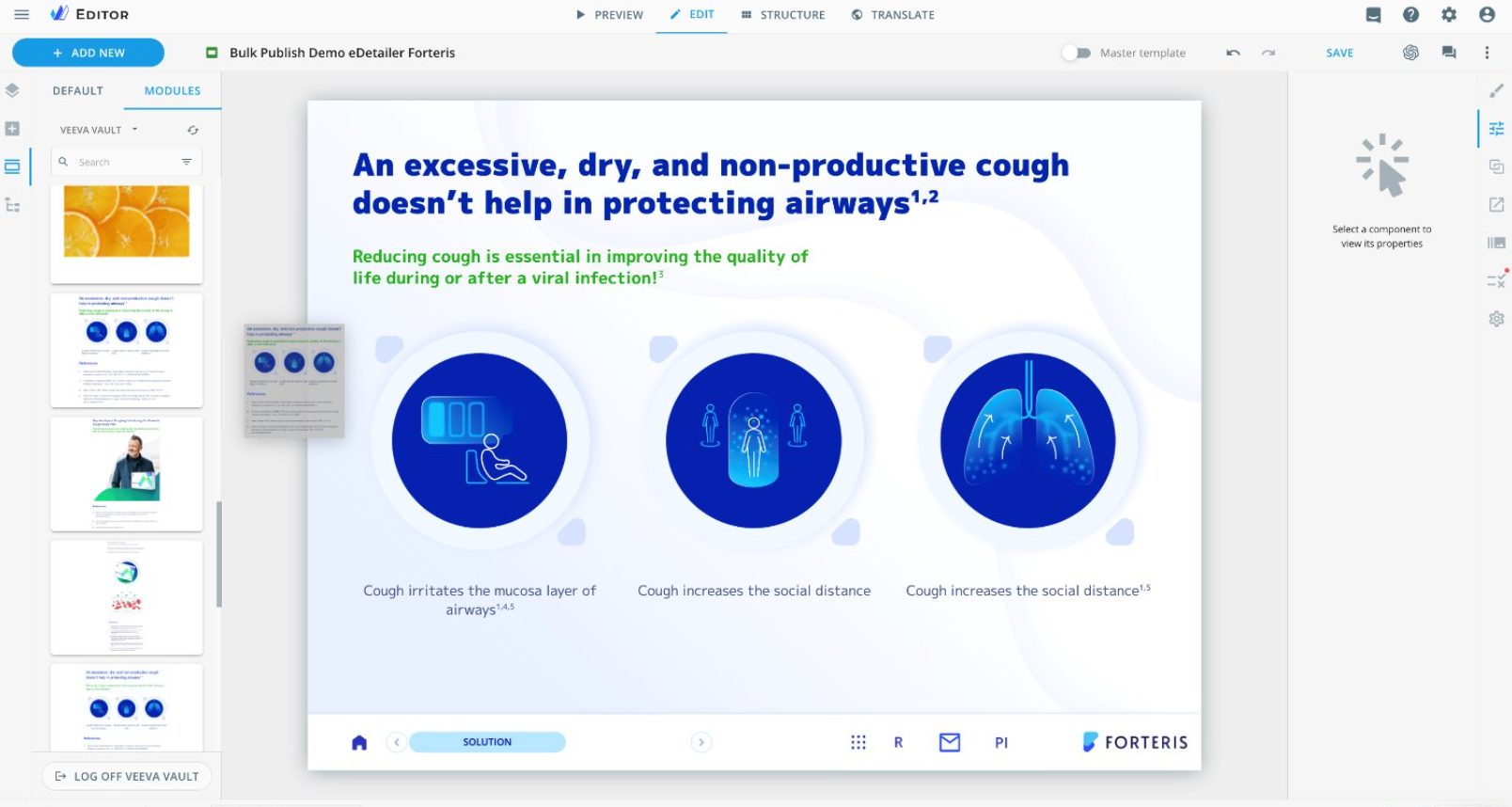
Universal block layout
Going all in
Whether you pursue an omnichannel strategy or implement AI, it is important to think big but take baby steps in your content production. Employees can easily feel overwhelmed and demotivated if you try to roll out all the technologies and processes simultaneously. Plus, it becomes tough to put a finger on what is working and what is holding you back.
So, take one content creation approach at a time so as not to risk organizational health. Get feedback from all stakeholders to identify areas that need improvement, support, and resources or to see if you are hitting your goals.
Future of Content Development
Engaging with customers in real time will be essential to keeping them loyal. With tech advancements and rising competition, customer preferences change fast. What is important today may not hold the same value tomorrow.
Brands are turning to AI-powered analytics to get real-time insights into their audience’s needs. Just like Amazon predicts what you will order next, pharma companies will create and approve content ahead of time, ensuring they deliver the most relevant information when needed.
The future is rooted in automation, but there is a caveat: you need to know what can be automated and still requires a human touch. In the drive for efficiency, it is vital to remember that full automation will not deliver the creativity or empathy needed to produce truly engaging content.
Customers want a genuine connection and to feel that a brand truly cares and is ready to go the extra mile. Ideally, your content would have to implicitly convey that.
Elevate Your Content Development Process with eWizard
Content development in life sciences is a long journey from the initial idea to the final release. Technology can facilitate this process, helping you eliminate many unnecessary steps. They empower brands to manage and make sense of their data, generate and localize assets, and speed up often-lengthy MLR approval stage.
eWizard is a platform built to make creating relevant, engaging content easy. You can choose from three options: outsource content development to our experts, use our self-service eWizard solutions, or opt for a hybrid approach, where you handle some tasks and leave the rest to us.
Last year, we were nominated for the Best Overall Content Marketing Software, and this year we have been recognized as a Top Pharma Marketing Solution Provider.
Interested in trying eWizard demo? You are just one button away from revolutionizing your content creation process.
